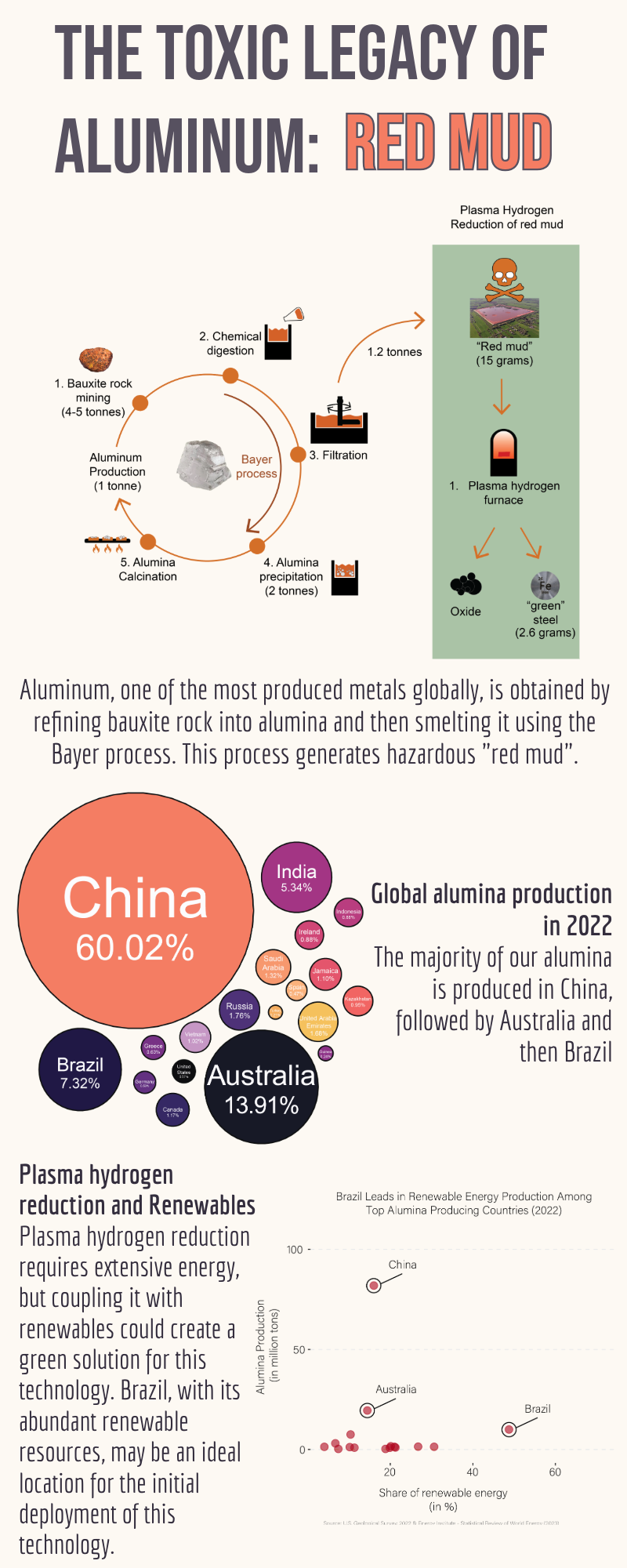
Aluminum production now has a greener way to deal with its waste. Aluminum is one of the most produced metals in the world. Lightweight and durable, it is a versatile material that can be used to create a wide variety of items, from electric cars to reusable lunch boxes. Aluminum has long been championed as a green and recyclable metal – but only if you overlook the tons of toxic byproducts produced each year during aluminum refinement.1 In a study published in Nature on January 24, chemist Matic Jovičević-Klug and colleagues at the Max Planck Institute for Sustainable Materials in Duesseldorf, Germany, discovered an exciting green solution to convert red mud into industrial building blocks: steel and oxide.2
The primary source of the world’s aluminum is bauxite rock. Each year, around 400 million metric tons of bauxite are extracted from large open-pit mines. To produce aluminum, the bauxite is first refined into alumina, which then gets smelted into aluminum. The alumina refinement leaves behind a toxic alkaline residue known as red mud. The hazardous red mud often gets dumped into landfills and concealed behind massive dam walls to be forgotten. However, as aluminum production continues to rise, it becomes increasingly difficult to ignore the staggering four billion tons of red mud that have accumulated. In addition to toxicity, dam failures are also a major concern surrounding red mud landfills, as evidenced by the 2010 red mud dam break in Ajka, Hungary. The break killed ten people, inflicted injuries on hundreds, and destroyed forty square kilometers of agricultural land—an area roughly half the size of Manhattan.
Red mud does come with a silver lining (or iron lining), however—it has high iron oxide content, which Jovičević-Klug and colleagues sought to extract and transform into pure steel using a novel plasma hydrogen reduction method they developed. The researchers placed the red mud into an electric arc furnace and exposed it to a plasma with 10% hydrogen, or ionized hydrogen gas. The plasma hydrogen in the electric furnace melts the liquid iron and liquid oxides in the red mud, which can then be more easily separated. In fifteen minutes, the reaction can extract as much as 2.6 grams of pure metallic iron from 15 grams of red mud.1 The resulting iron is so pure that it can be directly repurposed to produce steel, which can then be used in automobiles and packaging. An added bonus of the reaction is that it reduces the toxic alkalinity of the residual red mud to safe levels. This non-hazardous red mud can also be repurposed: for example, oxides are essential components in concrete, asphalt, paints, and various construction binders.
There is a clear benefit to using plasma hydrogen to reduce red mud: it yields a pure form of iron in a quick single-step process while simultaneously neutralizing the residual red mud.
However, the drawback is the substantial energy input required to power the furnace in which the reaction takes place. To overcome this bottleneck, the authors suggest using renewable energy to power the furnace, which would make the process carbon-neutral.
It is difficult to gauge how this technology could be scaled up without true cost analysis experiments. Countries like China, Australia, and Brazil, where most alumina refineries are located, stand to benefit the most from plasma hydrogen reduction.3 Renewables constitute over 14% of the total energy consumption in all three countries.4 Notably, the share of primary energy consumption in Brazil is 48%, making it a good candidate for the initial adoption of plasma hydrogen reduction.4 China, however, surpasses all other countries in alumina production, generating over 57% of the world’s alumina each year. This makes it an ideal place for the widespread implementation of scaled-up plasma hydrogen reduction.4 Plasma hydrogen reduction could provide a lucrative second life for billions of red mud landfills. With strategic investment, steel may become the healthy alumina waste of the future.
References:
- Svobodova-Sedlackova, Adela, et al. “Mapping the research landscape of bauxite by-products (Red Mud): An evolutionary perspective from 1995 to 2022.” Heliyon, vol. 10, no. 3, 15 Feb. 2024, https://doi.org/10.1016/j.heliyon.2024.e24943.
- Jovičević-Klug, M., Souza Filho, I.R., Springer, H. et al. Green steel from red mud through climate-neutral hydrogen plasma reduction. Nature 625, 703–709 (2024). https://doi.org/10.1038/s41586-023-06901-z
- U.S. Geological Survey, 2022, Mineral commodity summaries 2022: U.S. Geological Survey, 202 p., https://doi.org/10.3133/mcs2022.
- Energy Institute – Statistical Review of World Energy (2023) – with major processing by Our World in Data. “Share of primary energy consumption that comes from renewables – Using the substitution method” [dataset]. Energy Institute, “Statistical Review of World Energy” [original data]. https://ourworldindata.org/renewable-energy