Part IX: F. Peyton Rous, 1966 Prize in Physiology or Medicine
By Joseph Luna

“Whatever you do, don’t commit yourself to the cancer problem.” These were ominous words for a young pathologist named Peyton Rous to hear from his famed mentor William Welch. In the early 1900s, it seemed accurate. Cancer, then as now, is a terrifying constellation of diseases. This was all the more true in 1909, when few tools to study its deadly forms were available beyond the pathological descriptions afforded by the microscope. Added to this frustrating mix were scientific debates on the origins of cancer: some cancers were clearly inherited from one generation to the next, suggesting a genetic cause. And yet other cancers defied an inheritance rule and were instead closely associated with certain chemically-laden occupations, such as “soot wart” carcinoma among chimney sweepers. What if chemical exposures were the real culprit? In an era when chemical regulation was effectively non-existent for industrial workers, one can only imagine what Gilded Age employers would’ve thought of this theory. As a result, “cancer” was seen as a thorny and complex issue, only likely to become thornier. There seemed little a scientist could do to definitively address causes, let alone suggest treatment for cancer. Welch’s words were not far off the mark.
Yet, others were not as pessimistic. Simon Flexner, the Rockefeller Institute’s first director and also a student of Welch’s, offered Rous a position to take up the cancer problem, and Rous, despite some reluctance, went against his mentor’s advice and accepted the offer. Rous was hired ostensibly to take up studies of an epithelial tumor in rats known as the Flexner-Jobling tumor, notable in that it could be transplanted with some success between animals. The position, however, afforded the 31-year old pathologist considerable freedom to explore other potential models of cancer.
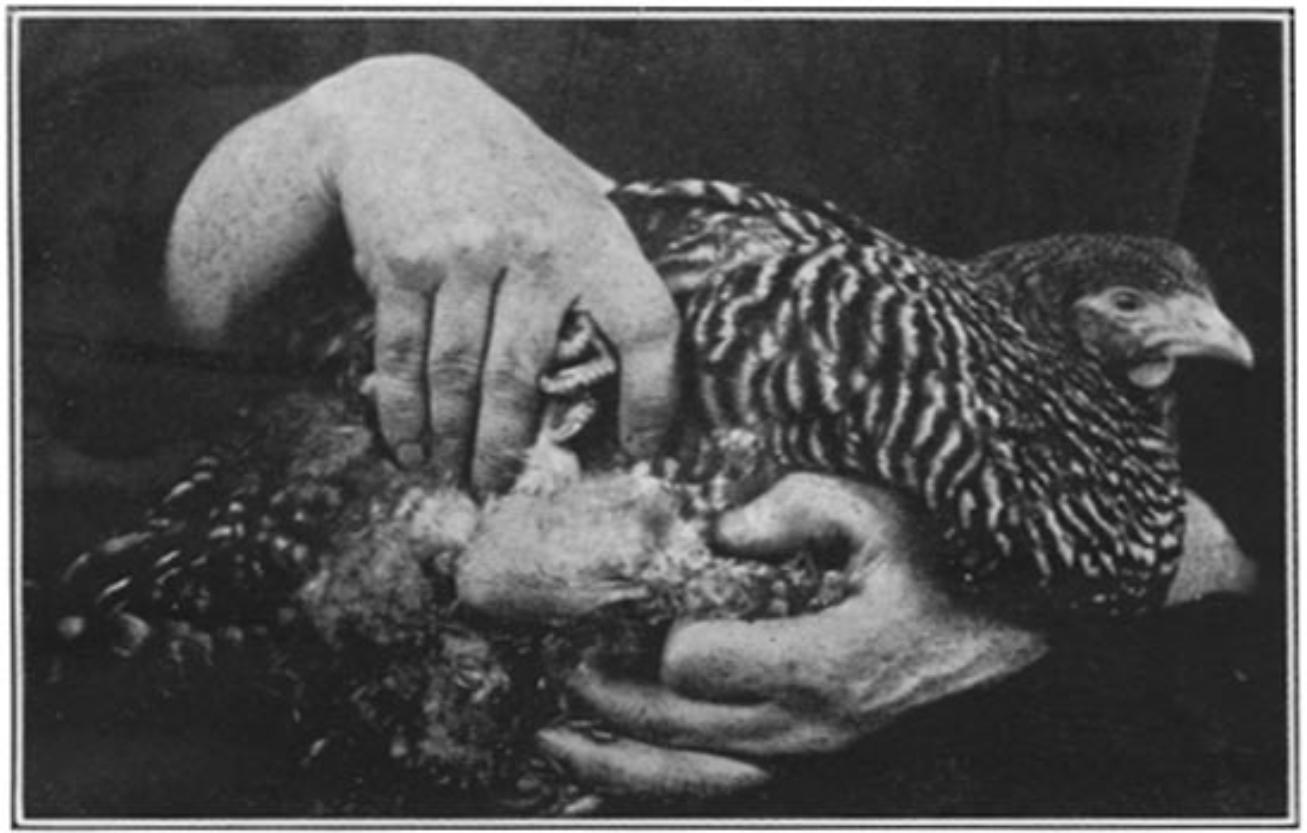
Soon after Rous got to work, at a time when live chickens were not an uncommon sight in Manhattan, one inquisitive poultry breeder brought to the institute a Plymouth Rock hen bearing a large tumor. We neither know what her precise motivations were to approach the new institute for medical research on Avenue A with a diseased chicken, nor do we know what Rous initially made of such a strange curiosity. But it was a chance and a fortuitous encounter. Rous took the chicken and attempted to do what many a would-be cancer researcher had tried but failed. After determining the type of cancer under the microscope, he attempted to transmit the tumor to a healthy bird. To his surprise, it worked. The once healthy bird developed tumors that looked almost exactly like the original. This work, published in 1910, established that a “sarcoma of the common fowl” could be transmitted. Such a model for cancer was an important first step in figuring out what caused it.
Rous next dove head-first into this causation problem. In an extraordinary hypothetical leap, Rous repeated his tumor transmission experiment with a twist. Instead of directly injecting bits of tumor into a bird, Rous first passed the tumor cells through a bacteria-tight filter and then injected a bird with the now cell-free filtrate. Scientific consensus of the day held that cancer, as a distinctly cellular phenomenon of “somatic mutations,” shouldn’t arise with injections of cell-free material. Yet within a few weeks, some of the injected birds developed tumors, though nothing was conclusive for Rous until he plied his trade at the microscope. Coming into focus, the methylene-blue and eosin stained tumor cells of bird number 177 almost shouted their answer: cancer. The spindle-cell sarcoma Rous observed in the new bird was indistinguishable from the tumor in the original hen. Rous had discovered that a filterable agent, in modern parlance a virus, could transmit cancer.
Published in 1911, the discovery of the first virus transmitted tumor caused quite a stir among cancer researchers, in that it demonstrated a viral origin in addition to suspected chemical and genetic causes of cancer. Without a unified theory of cancer, each of these three hypotheses was compelling individually, but appeared mutually incompatible with the others. Not surprisingly, few were convinced by the viral hypothesis in Rous’s day. The scientific establishment cried “contamination!” almost in unison upon reading his findings. Many doubted that Rous’s filtrates were completely devoid of living cells. When Rous and James B. Murphy freeze-dried the filtrate to ensure that all cells (if any) were killed and found that the filtrate was still tumorigenic, prominent researchers demonstrated that some cells could survive the freeze-drying treatment. No matter the suggestive evidence, there was always an alternate, if increasingly far-fetched, explanation. And for those few that believed Rous’s results, there was still the real concern of generality. Perhaps viral cancer transmission was a strange quirk of avian biology, and not applicable to more sophisticated mammalian tumors, which had eluded cell-free transmission. Rous’s own experience almost bore this out; he tried in vain for a few years to isolate a mammalian tumor virus. By the outbreak of World War I, he had moved on to other studies and shelved the project.
Shelved perhaps, but not forgotten. In 1933, more than two decades after the initial observation of a cancer-causing virus, Richard E. Shope from the Department of Animal Pathology at Princeton and a close friend of Rous, isolated a virus that caused wart-like growths in cottontail rabbits. While some might be hesitant to re-visit past work, Rous enthusiastically dove into the study of Shope’s papilloma virus, and within a year, reported from his Smith Hall laboratory that the warts were indeed true tumors. Over the ensuing decade until his retirement, Rous studied the Shope virus in great depth, proving its tumorigenic potential, its relations to other carcinogens, and characterizing its induced disease in no shortage of contexts. By the time Rous officially retired in 1945, it was clear that while not all cancers were viral in origin, a notable few certainly were.
Ultimate vindication wouldn’t arrive for yet another two decades, when in 1966, at the age of 87, Rous was finally awarded the Nobel Prize in Physiology or Medicine. It remains the longest “incubation period,” from discovery to prize, on record. ◉

